Abstract
The effects of CO2 and salt type on the interfacial tension (IFT) between crude oil and carbonated brine (CB) have not been fully understood. This study focuses on measuring the dynamic IFT between acidic crude oil with a total acid number of 1.5 mg KOH/g and fully CO2-saturated aqueous solutions consisting of 15,000 ppm of KCl, NaCl, CaCl2 and MgCl2 at 30 °C and a wide range of pressures (500–4000 psi). The results of IFT measurements showed that solvation of CO2 into all the studied aqueous solutions led to an increase in IFT of acidic crude oil (i.e., comparison of IFT of crude oil/CB and crude oil/brine), while no significant effect was observed for pressure. In contrast, the obtained results of studied salts indicated a positive effect on the IFT reduction of acidic crude oil/carbonated water (CW) (i.e., comparison of IFT of crude oil/CB and crude oil/CW).
Similar content being viewed by others
1 Introduction
In recent years, severe environmental problems and global warming, due to the accumulation of carbon dioxide (CO2) in the atmosphere, lead to the development of various technologies for the capturing and storage of CO2 (Abbaszadeh et al. 2016; Ahmadi et al. 2016a; Godec et al. 2013; Li et al. 2008; Zhao et al. 2010). In this way, injection of CO2 into petroleum reservoirs was proposed which can have the dual effect of global warming mitigation as well as oil recovery enhancement (Godec et al. 2013; Lashkarbolooki et al. 2016d; Luo et al. 2012; Riazi et al. 2011). For conventional CO2 injection, high mobility and gravity segregation lead to a low sweep efficiency and consequently lead to lower oil recovery performance (Dang et al. 2016; Shakiba et al. 2016; Sohrabi et al. 2012). In other words, the poor sweep efficiency of direct CO2 injection indicates a lower CO2 storage capacity as well as economically inefficient enhanced oil recovery process. To diminish these problems, an alternative scenario is the injection of CO2-enriched brine, i.e., carbonated brine (CB) (Lashkarbolooki et al. 2018; Riazi and Golkari 2016). Since CO2 is dissolved in the brine phase in CB injection and subsequently moves to the oil phase, rather than as a free gas phase during direct injection of CO2, a safer CO2 storage method is provided (Sohrabi et al. 2012). Moreover, since the injected CW is miscible with the formation of water, the trapped residual oil can easily come in contact with CO2 through mass transfer; this is further aided by the fact that the mobility of CW is much lower than that of gaseous CO2. The other desired reason of using carbonated water (CW) is that CW injection requires less CO2 than conventional CO2 injection (Riazi 2011).
Proposing CW injection for secondary and tertiary oil recovery processes dates back more than 60 years (Martin 1951; Saxon Jr et al. 1951). Unfortunately, due to its complex nature and considering economic factors, no serious efforts were undertaken based on this process (Holm 1959; Perez et al. 1992; Ramesh and Dixon 1973) till Riazi (2011) renewed the concept of carbonated water injection coupled with its capability not only for the production of higher oil recovery but also for the CO2 sequestration making it an interesting approach. Understanding the interfacial tension (IFT) behavior of crude oil/CO2-enriched brine is essential in ascertaining the migration of fluids in the reservoir. During the past decades, it has been widely reported that pressure had the most significant effect on IFT of crude oil/CO2 (Bayat et al. 2016; Lashkarbolooki and Ayatollahi 2018). It was found that the IFT of crude oil/CO2 dramatically declined with pressure until the minimum miscibility pressure (MMP) reached while the pressure had an insignificant effect on the IFT of crude oil/aqueous solution (Dong et al. 2001; Lashkarbolooki and Ayatollahi 2018). Among the investigated parameters, salinity showed the most important influence on the IFT of crude oil/aqueous phase, especially for crude oil containing significant natural active agents. The previous study revealed that IFT values of acidic crude oil/MgCl2 and CaCl2 aqueous solutions were lower compared with those obtained for deionized water (DW), NaCl and KCl solutions (Lashkarbolooki et al. 2014). It was also observed that, in this system, the divalent ions reduced the adsorption time more than monovalent ions (Lashkarbolooki and Ayatollahi 2017). As CO2 is dissolved in the brine phase, the IFT of crude oil/CB may significantly change compared to crude oil/CO2 and crude oil/brine systems. Based on the results reported by Johnson et al. (1952), Yang et al. (2005), Riazi and Golkari (2016) and Honarvar et al. (2017), it can be shown that the IFT of crude oil/CO2-enriched water is lower than that of crude oil/water which makes the IFT reduction a property of CB injection (Ahmadi et al. 2016b; Foroozesh et al. 2016; Mosavat and Torabi 2014). For instance, Riazi and Golkari (2016) and Honarvar et al. (2017) measured the IFT of crude oil/carbonated and noncarbonated brine. They reported that the CO2 had a positive effect on the IFT of crude oil/brine as the IFT of CB/crude oil was lower than the IFT of crude oil/normal brine because of the dissolution of CO2 in both crude oil and brine. They also stated that in the unadulterated brine, water molecules are oriented across the surface due to strong hydrogen bonds. In the carbonated system, CO2 molecules tend to transfer toward the crude oil/aqueous phase interface as a result of low reactivity toward polar H2O molecules. Reduction in the space available for H2O molecules and weakening the hydrogen bonds among them lead to an IFT reduction in the crude oil/carbonated system (Honarvar et al. 2017; Riazi and Golkari 2016). Honarvar et al. (2017) also observed that increasing pressure had a significant influence on the reduction in crude oil/CB IFT values since the solubility of CO2 increased in both phases. On the other hand, a comparison of the measured dynamic IFT values for two different types of crude oil by Lashkarbolooki et al. (2017a) revealed that acidic and/or basic ionizations of natural surfactants of the crude oil in the low pH values of carbonated water phase compared to the unadulterated phase were dominant parameters in the IFT. In other words, the IFT of crude oil/CW was a function of the crude oil type. Considering the effect of pH and total acid number of crude oil, they deduced that diffusion of CO2 in the crude oil hinders the movement and orientation of natural surfactants (especially acidic molecules) at the interface. Accordingly, IFT of acidic crude oil (ACO)/CW was considerably higher than a system with no CO2 content. Also no relationship was observed between the IFT of crude oil/CW and solubility of CO2 in the water phase (Lashkarbolooki et al. 2017a). In addition, Honarvar et al. (2017) observed lower IFT values of crude oil/carbonated sea water compared to carbonated formation brine. They have described this trend based on the higher solubility of CO2 in seawater compared to the formation of brine (Honarvar et al. 2017).
Since IFT of carbonated water/acidic crude oil has demonstrated unusual and confusing behavior, this study was designed as the continuation of our previous systematic procedure to find the effect of salt type on the dynamic IFT of ACO/CB. In this regard, the dynamic IFTs of ACO/CO2-enriched solutions consisting of 15,000 ppm of NaCl, KCl, CaCl2 and MgCl2 were measured and results were compared to those obtained for unadulterated brine without any CO2 content (Lashkarbolooki and Ayatollahi 2017). In addition, the adsorptions of the mentioned cases which were obtained based on the mono-exponential decay model were compared.
2 Experimental
2.1 Methods and materials
To investigate the effect of ion type on dynamic IFT of ACO/CW, several aqueous solutions consisting of 15,000 ppm of KCl, NaCl, MgCl2 and CaCl2 were prepared. All the salts were purchased from the Merck Company, Germany (purity > 99.9%). The heavy crude oil (API gravity of 21.5º) contained 11 wt% asphaltenes and 13 wt% resins with a total acid number of 1.5 mg KOH/g sample.
2.2 IFT measurement apparatus (Vinci technologies)
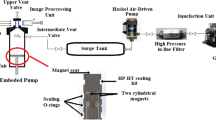
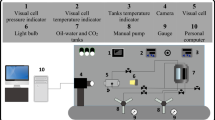
Axisymmetric drop shape analysis was used to measure the dynamic IFT of crude oil/aqueous phase. This is based on the change of crude oil drop shape on account of interface relaxation. First, a visual cell of the apparatus was filled with brine and fully saturated with CO2 until an equilibrium was reached at the desired pressure and temperature conditions which were, respectively, adjusted with a accuracy of 0.05 MPa by a manual pump and 0.1 K (with the assistance of a temperature controller). After the preparation of CB in the desired conditions in the visual cell, a crude oil drop was injected into the visual cell with the aid of a steel needle of 0.79 mm outer diameter. The shape of the crude oil drop was analyzed with online Vinci image processing software in order to calculate the dynamic IFT values. In addition, the equilibrium value was reported when no plateau in the IFT curve was observed. It should be noted that cleaning is one of the most important steps in the IFT measurement procedure. Before each IFT measurement, all parts of the equipment which are capable of being uninstalled were opened and soaked with toluene and n-hexane to remove all the contaminants. In addition, using a hand pump filled with toluene, all the other parts which cannot be uninstalled were rinsed carefully. After that, each part was again rinsed with n-hexane for more than three times to eliminate the contamination of toluene in this stage. After that, the system and its compartments were purged with compressed air to evaporate the n-hexane residue. The other point worth mentioning is that each reported equilibrium IFT value is the average of at least three independent measurements to not only find the most accurate value but also to check the repeatability of the performed procedure for IFT measurements.
3 Mono-exponential decay model
The mono-exponential decay model was applied to calculate the diffusion adsorption time (τ) from dynamic IFT (γ) (Jeribi et al. 2002; Lashkarbolooki et al. 2016a, b):
where t is time, s, and γ0 and γeq are the initial and equilibrium IFT, respectively. The adsorption/relaxation time provides a good idea of the characteristic time scale of the IFT variation (Jeribi et al. 2002).
4 Results and discussion
4.1 Evaluation of dynamic IFT of ACO/carbonated brine consisting of monovalent cation
Figure 1 shows the effect of pressure (P) on the dynamic IFT of crude oil and aqueous solutions consisting of 15,000 ppm of NaCl and KCl, which were fully saturated with CO2. In both cases, IFTs gradually decreased over time until becoming constant at the equilibrium state, for all the studied pressures. Equilibrium IFTs of both studied CBs decreased as the pressure increased from 500 to 1000 psi; however, they were unaffected by further pressure augmentation. Two concurrent reasons were postulated for the dynamic IFT reduction including the partition of CO2 molecules from the brine phase toward the oil phase and reorientation and packing of natural surfactants at the interface (Lashkarbolooki et al. 2017a, 2018). The acidic and/or basic natural surfactant ionization in the presence of brine and CO2 molecules at different CO2 contents may affect the packing of the active agents at the interface which stabilizes the oil/CB IFT. In more detail, a reduction in pH of the aqueous phase due to the existence of CO2 can increase or decrease the ionization of complex ions between basic or acidic natural surfactants and cations of the studied salts, respectively. To sum up, more ionization of natural surfactants may lead to more IFT reduction. To achieve further insight into the effect of CO2 on the IFT, dynamic IFT values between ACO and carbonated NaCl and KCl solutions (i.e., CNaCl and CKCl, 15,000 ppm) were compared at 30 °C and 4000 psi (see Fig. 2). Two different phenomena can be seen from dynamic IFT values of ACO/carbonated brine consisting of monovalent cations in the presence and absence of CO2. In comparison with NaCl and CNaCl, the initial IFT of carbonated brine was more than the straight brine. In addition, the decline in the dynamic IFT of CNaCl was less than that of NaCl which led to a considerable enhancement in the equilibrium IFT of CNaCl compared to NaCl. However, for solutions consisting of KCl, a contradictory pattern was observed. The initial IFT of KCl was more than that of CKCl while a reduction in IFT over time was more considerable for KCl which shifted both systems toward almost the same equilibrium IFT values.
One of the most important parameters which may affect the interfacial behavior of the studied systems is the acidity of the aqueous phase due to the dissolution of CO2 which can reduce the pH of the aqueous phase to about 3 (Ross 1982; Toews et al. 1995). To separate the direct effect of CO2 molecules at the interface and its side effect regarding pH reduction, the pH of the aqueous solutions was adjusted to 3.5 using 0.1 M HCl solution and IFTs between the obtained solutions and ACO were measured. The obtained results showed that the reduction in the brine pH had a reverse effect on the equilibrium IFT of 15,000 ppm NaCl and KCl solutions to about 1.8 and 4.4 mN/m, respectively. On the other hand, in the absence of CO2, the pH reduction reduced the ionization of acidic natural surfactants which subsequently increased the IFT. If the ionization of natural surfactants was the most significant mechanism, the equilibrium IFT between the KCl solution and ACO should have experienced higher IFT enhancement in the presence of carbonated brine; however, this phenomenon was not observed. These observations proved the competition between ionization of natural surfactants at low pH values and partition of CO2 from brine toward ACO phases as well as their interfacial interactions which can stabilize the IFT of brine and ACO.
4.2 Evaluation of dynamic IFT of ACO/carbonated brine consisting of divalent cations
Dynamic IFTs of ACO/CB consisting of either 15,000 ppm of MgCl2 or CaCl2 are shown in Fig. 3. These figures reveal that for both systems and all the studied pressure conditions, the IFT steadily decreased and finally reached the equilibrium state. As it is well known, increasing pressure makes it possible to solubilize CO2 molecules in the aqueous phase (see Fig. 4) and their partitioning into the crude phase results in crude oil swelling (Lashkarbolooki et al. 2017b, 2018; Yang et al. 2005). However, the effect of pressure which supplied from the injected CO2 on the water and consequently CO2 content in the brine phase did not show a remarkable IFT variation for solutions containing divalent cations. The effect of CO2 on the dynamic IFT of carbonated brine consisted of 15,000 ppm of CaCl2 or MgCl2 (i.e., CCaCl2 or CMgCl2) was investigated by comparing the measured dynamic IFTs with the results of a previous study (Lashkarbolooki and Ayatollahi 2017), which was performed for the same crude oil, brine and under similar operating conditions (i.e., temperature T = 30 °C and pressure P = 1000 psi) (see Fig. 5). At these conditions, equilibrium IFT values of 15000 ppm of CaCl2 (16.5 mN/m) and MgCl2 (6.8 mN/m) solutions were less than those of deionized water (21.9 mN/m). The results of Fig. 5 also demonstrated that the initial IFT values of both carbonated brines increased in comparison with straight brine. It can be deduced that CO2 decreased the affinity of natural surfactants (i.e., asphaltene and resin) toward the interface of crude oil and salts consisting of divalent cations. In addition, for both studied brines containing divalent cations, the IFT reduction in the brine phase over time was more noticeable compared to the carbonated brine which led to a remarkable equilibrium IFT value of crude oil/CB compared to the IFT of crude oil/brine. The formation of complex ions between polar natural surfactants and divalent cations of MgCl2 and CaCl2 (Lashkarbolooki et al. 2014), which improves their orientation and packing at the crude oil/brine interface, could be one of the possible reasons for this observation. Comparing the IFT values measured for ACO and 15000 ppm of MgCl2 and CaCl2 at low pH value (i.e., 3.5) and neutral conditions showed an increase of about 19.1 and 7.0 mN/m in IFT values for the acidic condition.
Solubility of CO2 in the studied solutions at different pressures and 30 °C (calculated based on Duan et al. (2006) model)
Therefore, it was confirmed that orientation and packing of natural surfactants disturbed at low pH values were likely due to the interruption of complex ions and the reduction in ionization of acidic components of ACO (with TAN of 1.5 mg KOH/g). In summary, it was deduced that the addition of CO2 which reduced the acidity of the aqueous phase due to the formation of carbonic acid (Riazi 2011) caused the interruption of complex ions between acidic natural surfactants and divalent cations of Ca2+ and Mg2+, consequently causing a significant increase in IFT values of ACO/CB compared to IFT of ACO/brine solution.
4.3 Equilibrium IFT evaluation of ACO/CB and ACO/brine
The impacts of pressure and salt type on the equilibrium IFT of ACO/CB were evaluated and are listed in Table 1. The effect of salt type was more obvious than the effect of pressure. Generally, a negligible IFT reduction was observed as the pressure increased from 500 to 4000 psi for all the studied salts while no specific pattern was observed for equilibrium IFT of ACO/CB. To clear up the effect of salt type on the IFT, variations between equilibrium IFTs of CW and CB (i.e., \(\gamma_{{{\text{eq,}}\;{\text{CW}}}} - \gamma_{{{\text{eq,}}\;{\text{CB}}}}\)) as well as those of brine and CB (i.e., \(\gamma_{{{\text{eq,}}\,\;{\text{B}}}} - \gamma_{{{\text{eq,}}\,\;{\text{CB}}}}\)) are shown in Fig. 6a, b. In the case of the carbonated system (Fig. 6a), the addition of salts decreased the IFT of CW/ACO while the addition of CO2 to the brine solutions increased the IFT values (see Fig. 6b) without their being noticeably affected by pressure. As shown in Fig. 6b, the solution containing K+ (i.e., KCl) showed less IFT variation in the presence of CO2 than the solution containing Na+ (i.e., NaCl). It is worth noting that the hydrated radius and enthalpy of hydration of K+ (232 pm and −340 kJ/mol) are less than those of Na+ (276 pm and −424 kJ/mol) and the apparent charge and ionic mobility of K+ (0.82 and 64.5 cm2/ohm mol) are higher than those of Na+ (0.79 and 43.5 cm2/ohm mol) (Barrett 2003; Lashkarbolooki et al. 2017b). Accordingly, polar natural surfactants had more accessibility to K+ compared to Na+ which led to better packing of surface active agents in the presence of CO2 molecules and K+ cations. On the other hand, asphaltene and resin molecules which contain heteroatoms in their structures specially O2− showed high affinity to Mg2+ cations and the formation of complex ions between them in the neutral conditions (Lashkarbolooki et al. 2014, 2016c). However, the reduction in the pH of the aqueous phase due to the addition of CO2 likely reduced this affinity and consequently enhanced the IFT to its ultimate value for carbonated brine consisting of 15000 ppm of MgCl2. To sum up, the lowest and highest IFT variations in the presence of CO2 were obtained for KCl and MgCl2, respectively.
4.4 Adsorption time evaluation of ACO/CB and ACO/brine
The obtained adsorption times of all the studied carbonated aqueous phases are shown in Fig. 7. For all the studied cases, a reduction in adsorption time can be observed as the pressure increased, likely due to the enhancement of entropy and molecular movement in the presence of CO2 molecules. Furthermore, the influence of pressure on the adsorption time of the carbonated aqueous phase is depicted in Fig. 8a. The effect of pressure on the adsorption time of the carbonated brine was more considerable in comparison with carbonated water. Therefore, the \(\tau_{\text{CW}} - \tau_{\text{CB}}\) increased as the pressure increased. In addition, the effect of CO2 on the adsorption time of ACO/CB is shown in Fig. 8b. The results shown in this figure reveal that the presence of CO2 increased the adsorption time of the carbonated phase, especially for the aqueous solution consisting divalent cations, likely due to complex ions formed between asphaltenes and resins of acidic crude oil and divalent cations although it loosed at low pH values of the carbonated phase. Therefore, their affinity toward the interface decreased which enhanced the time required to reach the equilibrium condition.
5 Conclusions
The results of dynamic interfacial tension measurements along with dynamic mono-exponential decay modeling of the IFT values for the crude oil consisting of acidic asphaltene and resin fractions and carbonated brine with 15000 ppm of chloride salts including monovalent (i.e., Na+ and K+) and divalent cations (i.e., Mg2+ and Ca2+) are summarized as follows:
-
The acidity of the aqueous phase due to the dissolution of CO2 can reduce the ionization of acidic components of ACO and disturb the proper packing of them at the interface. This phenomenon can increase IFT values of ACO and CB in comparison with unadulterated solution, especially for the salts consisting of divalent cations.
-
The higher IFT value of ACO/CMgCl2 compared with the IFT of unadulterated brine solution supports the idea that the high affinity of Mg2+ cations toward natural acidic surfactants in the crude oil can be broken at the low pH value of carbonated brine.
-
The effect of salt type on the IFT of ACO/CB is more noticeable compared to the effect of pressure. In detail, the addition of CO2 to the aqueous phase increases the IFT of ACO in the following order: MgCl2 > DW ≥ NaCl ≥ CaCl2 > KCl. In addition, pressure enhancement from 500 psi to 1000 psi leads to an increase in \(\gamma_{{{\text{eq,}}\;{\text{CW}}}} - \gamma_{{{\text{eq,}}\;{\text{CB}}}}\) while further increase in pressure to 4000 psi introduces no significant effect.
-
The addition of salts to the carbonated aqueous phase decreases the IFT of ACO/CW while the addition of CO2 to the brine solutions increases the IFT values in comparison with straight brine solutions.
-
The presence of CO2 leads to an increase in the adsorption time of carbonated phases, especially for aqueous solutions containing divalent cations.
Abbreviations
- ACO:
-
Acidic crude oil
- CB:
-
Carbonated brine
- CCaCl2 :
-
15,000 ppm of carbonated CaCl2 solution
- CKCl:
-
15,000 ppm of carbonated KCl solution
- CMgCl2 :
-
15,000 ppm of carbonated MgCl2 solution
- CNaCl:
-
15,000 ppm of carbonated NaCl solution
- CW:
-
Carbonated water
- DW:
-
Deionized water
- MMP:
-
Minimum miscibility pressure
- SD:
-
Standard deviation
- t :
-
Time (s)
- τ :
-
Adsorption time (s)
- γ 0 :
-
Initial IFT
- γ eq :
-
Equilibrium IFT
References
Abbaszadeh M, Nasiri M, Riazi M. Experimental investigation of the impact of rock dissolution on carbonate rock properties in the presence of carbonated water. Environ Earth Sci. 2016;75(9):791. https://doi.org/10.1007/s12665-016-5624-3.
Ahmadi MA, Pouladi B, Barghi T. Numerical modeling of CO2 injection scenarios in petroleum reservoirs: application to CO2 sequestration and EOR. J Nat Gas Sci Eng. 2016a;30:38–49. https://doi.org/10.1016/j.jngse.2016.01.038.
Ahmadi MA, Hasanvand M, ShokrollahzadehBehbahani S, Nourmohammad A, Vahidi A, Amiri M, et al. Effect of operational parameters on the performance of carbonated water injection: experimental and numerical modeling study. J Supercrit Fluids. 2016b;107:542–8. https://doi.org/10.1016/j.supflu.2015.07.012.
Barrett J. Inorganic chemistry in aqueous solution, vol. 21. Cambridge: Royal Society of Chemistry; 2003.
Bayat M, Lashkarbolooki M, Hezave AZ, Ayatollahi S. Investigation of gas injection flooding performance as enhanced oil recovery method. J Nat Gas Sci Eng. 2016;29:37–45. https://doi.org/10.1016/j.jngse.2015.12.047.
Dang C, Nghiem L, Nguyen N, Chen Z, Nguyen Q. Evaluation of CO2 low salinity water-alternating-gas for enhanced oil recovery. J Nat Gas Sci Eng. 2016;35:237–58. https://doi.org/10.1016/j.jngse.2016.08.018.
Dong M, Huang S, Dyer SB, Mourits FM. A comparison of CO2 minimum miscibility pressure determinations for Weyburn crude oil. J Pet Sci Eng. 2001;31(1):13–22. https://doi.org/10.1016/S0920-4105(01)00135-8.
Duan Z, Sun R, Zhu C, Chou IM. An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO4 2−. Mar Chem. 2006;98(2–4):131–9. https://doi.org/10.1016/j.marchem.2005.09.001.
Foroozesh J, Jamiolahmady M, Sohrabi M. Mathematical modeling of carbonated water injection for EOR and CO2 storage with a focus on mass transfer kinetics. Fuel. 2016;174:325–32. https://doi.org/10.1016/j.fuel.2016.02.009.
Godec ML, Kuuskraa VA, Dipietro P. Opportunities for using anthropogenic CO2 for enhanced oil recovery and CO2 storage. Energy Fuels. 2013;27(8):4183–9. https://doi.org/10.1021/ef302040u.
Holm L. Carbon dioxide solvent flooding for increased oil recovery. Pet Trans AIME. 1959;216:225–31.
Honarvar B, Azdarpour A, Karimi M, Rahimi A, Afkhami Karaei M, Hamidi H, et al. Experimental investigation of interfacial tension measurement and oil recovery by carbonated water injection: a case study using core samples from an Iranian carbonate oil reservoir. Energy Fuels. 2017;31(3):2740–8. https://doi.org/10.1021/acs.energyfuels.6b03365.
Jeribi M, Almir-Assad B, Langevin D, Henaut I, Argillier J. Adsorption kinetics of asphaltenes at liquid interfaces. J Colloid Interface Sci. 2002;256(2):268–72. https://doi.org/10.1006/jcis.2002.8660.
Johnson W, Macfarlane R, Breston J, Neil D. Laboratory experiments with carbonated water and liquid carbon dioxide as oil recovery agents. Prod Mon. 1952;17:18–22.
Lashkarbolooki M, Ayatollahi S. Experimental and modeling investigation of dynamic interfacial tension of asphaltenic-acidic crude oil/aqueous phase containing different ions. Chin J Chem Eng. 2017;25(12):1820–30. https://doi.org/10.1016/j.cjche.2017.02.004.
Lashkarbolooki M, Ayatollahi S. Experimental investigation on CO2-light crude oil interfacial and swelling behavior. Chin J Chem Eng. 2018;26(2):373–9. https://doi.org/10.1016/j.cjche.2017.07.010.
Lashkarbolooki M, Ayatollahi S, Riazi M. Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels. 2014;28(11):6820–9. https://doi.org/10.1021/ef5015692.
Lashkarbolooki M, Ayatollahi S, Riazi M. Mechanistic study on the dynamic interfacial tension of crude oil + water systems: experimental and modeling approaches. J Ind Eng Chem. 2016a;35:408–16. https://doi.org/10.1016/j.jiec.2016.01.025.
Lashkarbolooki M, Riazi M, Ayatollahi S. Investigation of effects of salinity, temperature, pressure, and crude oil type on the dynamic interfacial tensions. Chem Eng Res Des. 2016b;115:53–65. https://doi.org/10.1016/j.cherd.2016.09.020.
Lashkarbolooki M, Riazi M, Ayatollahi S, Hezave AZ. Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel. 2016c;165:75–85. https://doi.org/10.1016/j.fuel.2015.10.030.
Lashkarbolooki M, Vaezian A, Hezave AZ, Ayatollahi S, Riazi M. Experimental investigation of the influence of supercritical carbon dioxide and supercritical nitrogen injection on tertiary live-oil recovery. J Supercrit Fluids. 2016d;117:260–9. https://doi.org/10.1016/j.supflu.2016.07.004.
Lashkarbolooki M, Riazi M, Ayatollahi S. Effect of CO2 and natural surfactant of crude oil on the dynamic interfacial tensions during carbonated water flooding: experimental and modeling investigation. J Pet Sci Eng. 2017a;159:58–67. https://doi.org/10.1016/j.petrol.2017.09.023.
Lashkarbolooki M, Ayatollahi S, Riazi M. Mechanistical study of effect of ions in smart water injection into carbonate oil reservoir. Process Saf Environ Prot. 2017b;105:361–72. https://doi.org/10.1016/j.psep.2016.11.022.
Lashkarbolooki M, Riazi M, Ayatollahi S. Experimental investigation of dynamic swelling and Bond number of crude oil during carbonated water flooding; Effect of temperature and pressure. Fuel. 2018;214:135–43. https://doi.org/10.1016/j.fuel.2017.11.003.
Li X, Hou M, Zhang Z, Han B, Yang G, Wang X, et al. Absorption of CO2 by ionic liquid/polyethylene glycol mixture and the thermodynamic parameters. Green Chem. 2008;10(8):879–84. https://doi.org/10.1039/B801948G.
Luo S, Xu R, Jiang P. Effect of reactive surface area of minerals on mineralization trapping of CO2 in saline aquifers. Pet Sci. 2012;9(3):400–7. https://doi.org/10.1007/s12182-012-0224-7.
Martin J. Additional oil production through flooding with carbonated water. Prod Mon. 1951;18.
Mosavat N, Torabi F. Experimental evaluation of the performance of carbonated water injection (CWI) under various operating conditions in light oil systems. Fuel. 2014;123:274–84. https://doi.org/10.1016/j.fuel.2014.01.077.
Perez J, Poston S, Sharif Q. Carbonated water imbibition flooding: an enhanced oil recovery process for fractured reservoirs. In: SPE/DOE enhanced oil recovery symposium, 22–24 April, Tulsa, Oklahoma; 1992. https://doi.org/10.2118/24164-MS.
Ramesh A, Dixon T. Numerical simulation of carbonated waterflooding in a heterogeneous reservoir. In: SPE symposium on numerical simulation of reservoir performance, 11–12 January, Houston, Texas; 1973. https://doi.org/10.2118/4075-MS.
Riazi M. Pore scale mechanisms of carbonated water injection in oil reservoirs. Doctoral dissertation, Heriot-Watt University; 2011.
Riazi M, Golkari A. The influence of spreading coefficient on carbonated water alternating gas injection in a heavy crude oil. Fuel. 2016;178:1–9. https://doi.org/10.1016/j.fuel.2016.03.021.
Riazi M, Sohrabi M, Jamiolahmady M. Experimental study of pore-scale mechanisms of carbonated water injection. Transp Porous Med. 2011;86(1):73–86. https://doi.org/10.1007/s11242-010-9606-8.
Ross GD. The dissolution effects of carbonated water on oil reservoir carbonates: a study under high pressure carbon dioxide flood conditions. Doctoral dissertation, Heriot-Watt University; 1982.
Saxon J Jr, Breston J, Macfarlane R. Laboratory tests with carbon dioxide and carbonated water as flooding mediums. Prod Mon. 1951;16:8–17.
Shakiba M, Ayatollahi S, Riazi M. Investigation of oil recovery and CO2 storage during secondary and tertiary injection of carbonated water in an Iranian carbonate oil reservoir. J Pet Sci Eng. 2016;137:134–43. https://doi.org/10.1016/j.petrol.2015.11.020.
Sohrabi M, Kechut NI, Riazi M, Jamiolahmady M, Ireland S, Robertson G. Coreflooding studies to investigate the potential of carbonated water injection as an injection strategy for improved oil recovery and CO2 storage. Transp Porous Med. 2012;91(1):101–21. https://doi.org/10.1007/s11242-011-9835-5.
Toews KL, Shroll RM, Wai CM, Smart NG. pH-defining equilibrium between water and supercritical CO2: influence on SFE of organics and metal chelates. Anal Chem. 1995;67(22):4040–3. https://doi.org/10.1021/ac00118a002.
Yang D, Tontiwachwuthikul P, Gu Y. Interfacial tensions of the crude oil + reservoir brine + CO2 systems at pressures up to 31 MPa and temperatures of 27 °C and 58 °C. J Chem Eng Data. 2005;50(4):1242–9. https://doi.org/10.1021/je0500227.
Zhao H, Liao X, Chen Y, Zhao X. Sensitivity analysis of CO2 sequestration in saline aquifers. Pet Sci. 2010;7(3):372–8. https://doi.org/10.1007/s12182-010-0080-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Yan-Hua Sun
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lashkarbolooki, M., Hezave, A.Z. & Ayatollahi, S. The role of CO2 and ion type in the dynamic interfacial tension of acidic crude oil/carbonated brine. Pet. Sci. 16, 850–858 (2019). https://doi.org/10.1007/s12182-019-0310-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12182-019-0310-1